Cortical and thalamic innervation of striatal interneurons
Htr3a interneurons
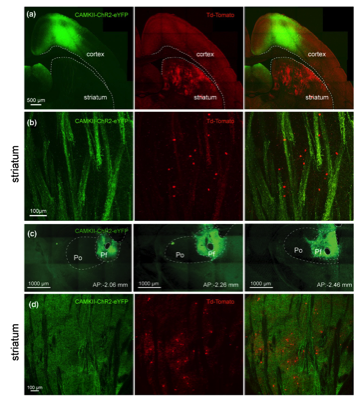
(a) Confocal image of cortical neurons transfected with a CAMKII-ChR2 -EYFP Adeno-associated virus (AAV, green) and Htr3a-Cre -transduced interneurons following striatal AAV-Flex -tdTomato injection (red) in a Htr3a transgenic mouse (oblique parahorizontal plane). (b) Higher magnification confocal imageshowing corticostriatal fibers in green and Htr3a-Cre -transduced interneurons in red. (c) Confocal image of the AAV-CAMKII-ChR2 -EYFP injection site in the PfN at 3 anteroposterior anatomical levels (−206, −2.26 and −2.46 from bregma). (d) Confocal image of Htr3a-Cre transduced interneurons (red) and thalamostriatal axons (green, coronal section).
Within the cerebral cortex, neurons expressing PV, SOM, or the ionotropic serotonin receptor, 5HT3a, constitute almost the entire interneuronal population (Rudy et al., 2011). This prompted the creation of a BAC transgenic mouse line expressing Cre under the control of the 5HT3a (Htr3a) promoter. This mouse line has been extremely useful for the study of striatal interneurons and thus far has allowed for the discovery of 2 novel striatal GABAergic interneurons that do not express previously described striatal GABAergic interneuronal markers (e.g., PV, SOM, NOS, TH), and for which the physiology, morphology and connections of these targeted interneurons type have been at least initially described.
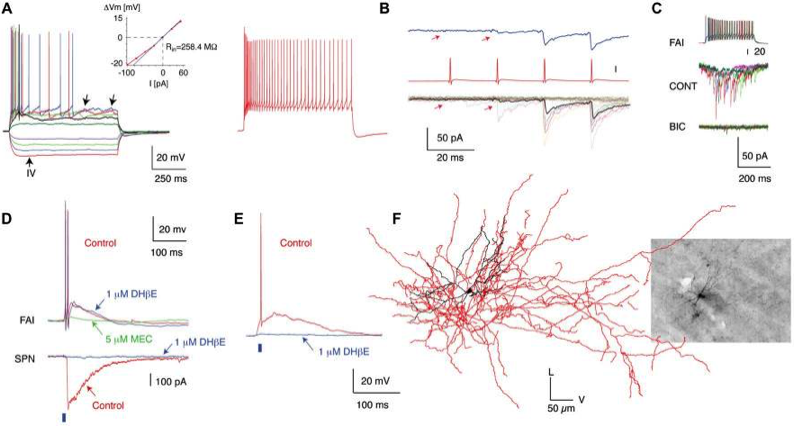
Fast-Adapting Interneurons (FAIs)
The first novel interneuron was the Fast-Adapting Interneuron (FAI). (A) Membrane potential responses of a typical FAI. Note pronounced spike frequency adaptation and irregular membrane potential fluctuations (left panel, arrows). Inset shows current–voltage relationship. (B) IPSC trains in an SPN elicited by trains of presynaptic action potentials in a FAI. (C) IPSCs in SPNs are blocked by a GABAA receptor antagonist (bicuculline, 10 mM). (D) Simultaneous recording from an FAI and an SPN. Optogenetic activation of cholinergic inputs (2-ms pulse of blue light, blue bar) elicited a large-amplitude IPSC in the SPN (bottom) and an EPSP giving rise to action potentials in the FAI (top). While application of DHbE (1 mM) blocks the IPSC in SPNs and the nicotinic EPSP in some FAI (E) it has no effect on other FAI (D; upper traces). The nicotinic EPSP is blocked by MEC (5 mM). (F) 3D reconstruction of a FAI filled with biocytin after recording (Inset). The soma and dendritic fields are represented in black and the axon in red in the reconstructed image. Data from Faust et al (2015) Eur. J. Neurosci.doi:10.1111/ejn.12915
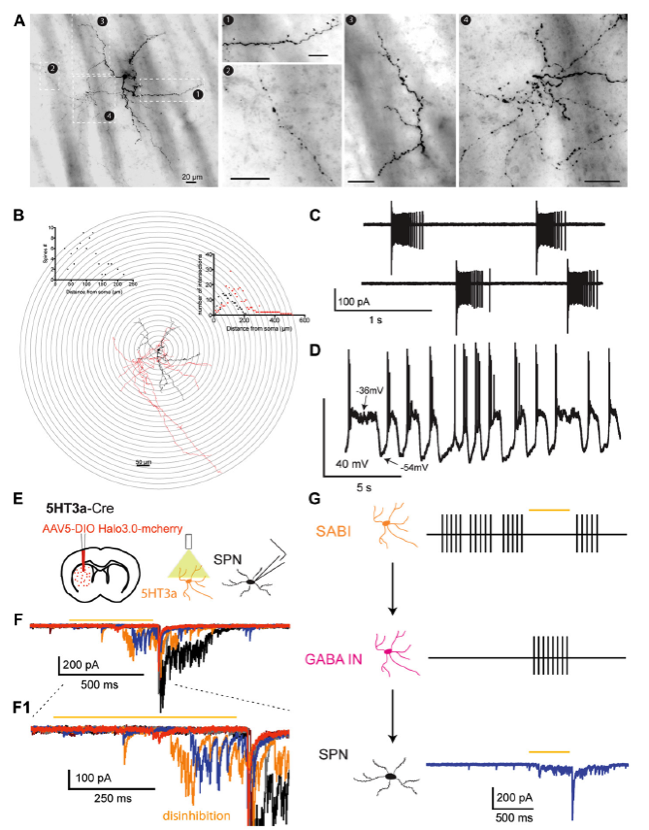
Spontaneously Active Bursty Interneurons (SABIs)
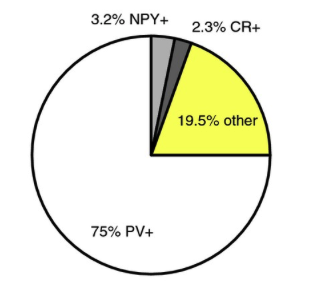
The largest population of striatal neurons targeted in the Htr3a-Cre mouse proved to be PV+ fast-spiking interneurons. Smaller proportions turned out to be NPY+ , but only NGF interneurons, not LTS interneurons, and another small percentage were CR+ interneurons. But there were almost 20% that were not stain for any known striatal peptides. From this 20%, we identified two novel GABAergic interneurons. See Faust et al (2015) Eur. J. Neurosci.doi:10.1111/ejn.12915
A. Neurocytology of electrophysiologically identified biocytin-labeled SABI interneurons. B. 3D reconstruction and Sholl analysis of SABIs. The soma and dendritic fields are represented in black and the axon in red in the reconstructed image and the associated Sholl plot. C. Cell-attached recording of a SABI. Note extreme the typical extreme burst firing, separated by periods of complete silence. D. Spontaneous firing activity recorded in approximately half of SABI exhibit membrane potential fluctuations with action potential firing happening only during the beginning of the dep/olarized state. E. AAV5 Ef1a DIO HR3.0-EYFP was injected into the striatum of Htr3a-Cre mice and SPNs were recorded ex vivo using a cesium-based high-chloride internal solution (125mM CsCl). F1. Representative examples of raw voltage-clamp data traces recordings in a SPN in the disinhibition protocol. Orange bar indicates the yellow light pulse. F2, Expanded view of the disinhibitory IPSCs occurring during and immediately after the yellow light pulse. G. Circuit diagram depicting the disinhibitory circuit hypothesized to mediate these responses. The Htr3a-Cre interneurons that are spontaneously active (i.e., the SABIs) are inhibited by halorhodopsin, which in turn disinhibits another, as yet unidentified population of interneuron(s) evoking these IPSC barrages in SPN. (Adapted from Assous et al., 2018).